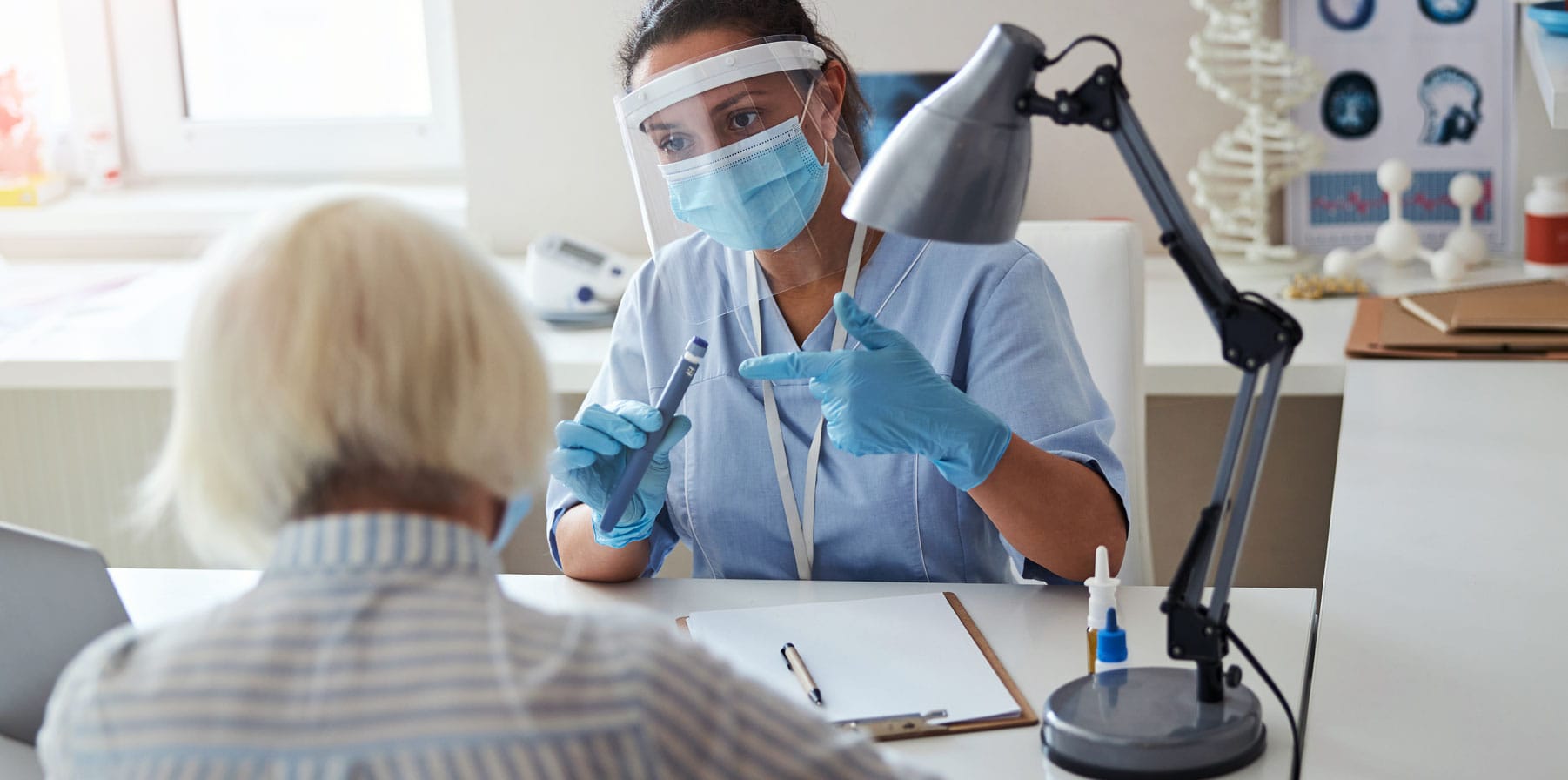
In-depth Drug Delivery Device Expertise
Phillips Medisize has the expertise and experience to create design strategies for all stages of development, from clinical trials to small- and large-scale commercial production. With 23 manufacturing facilities and seven R&D innovation centers located across the US, Europe and Asia, we collaborate and deliver to our pharmaceutical customers wherever they are.
We take a customer-centric, engineering-driven approach to innovation and production. In doing so, we cover every aspect of your journey from ideation and feasibility to development, clinical trials, scaled manufacturing, and finished device assembly, including advanced packaging, drug handling and cold storage management.
Whether you need assistance developing a bespoke device, utilizing one of our drug delivery platforms, or scaling the manufacturing of your existing device design, we can help you bring your biopharma products to healthcare providers and their patients faster. We are privileged to assist you in delivering treatments that enhance and save lives.
Additionally, for inhalation and nasal routes of administration, the 2025 Vectura acquisition expanded our capabilities. Together we now offer formulation, device design and combination product development, as well as manufacturing services to support the growing global need for inhalation and nasal therapies. Our expertise includes small molecules, biologics, complex combinations and generic products for pMDI, DPI, nasal inhaler and nebulization devices.
Our customers include 80 percent of the top 10 most valuable pharmaceutical companies.1 In 2024, we helped our biopharma customers:
Develop
441M+
Lifesaving and Life-enhancing Drug Delivery Devices
Care for
32M
Insulin-dependent Patients
Deliver
3.4M
Emergency Doses of Lifesaving Epinephrine
Advanced Manufacturing Capabilities for Drug Delivery Systems

Autoinjectors

Pen Injectors

Inhalation Devices

Ocular Drug Delivery Systems

Wearable On-Body Injectors, Syringe Systems and Needle Safety Devices
PHARMA-SPECIFIC DESIGN AND DEVELOPMENT CAPABILITIES
- Bespoke design leveraging innovative proprietary platform technologies
- Human-centered design, including human factors engineering (HFE) and user experience (UX)
- Mechanical, electromechanical and materials engineering
- Design assurance and design history file (DHF) support
- Test engineering and design validation testing (DVT)
- Scalable tooling and assembly expertise
- Platform devices and connected health solutions
- Clinical device manufacturing

INHALATION AND NASAL CAPABILITIES
PHARMA DEVICE MANUFACTURING SERVICES
- Controlled manufacturing sites
- Complex, highly sophisticated injection molding
- Bespoke manufacturing strategies for each customer
- Advanced and fully automated assembly technologies
- Final assembly, packaging and labeling services, including cold chain storage, serialization and unique device identification (UDI)
- Legal manufacturing and post-launch regulatory services
- Access to the extended Molex network of industry-leading electromechanical capabilities
- Advanced global supply chain tools and capabilities

Molex, a parent to Phillips Medisize, Closes Acquisition of Vectura Group Ltd. Learn more.
Featured Resources



1. Healthcare 2024. Brand Finance. Accessed November 2024.