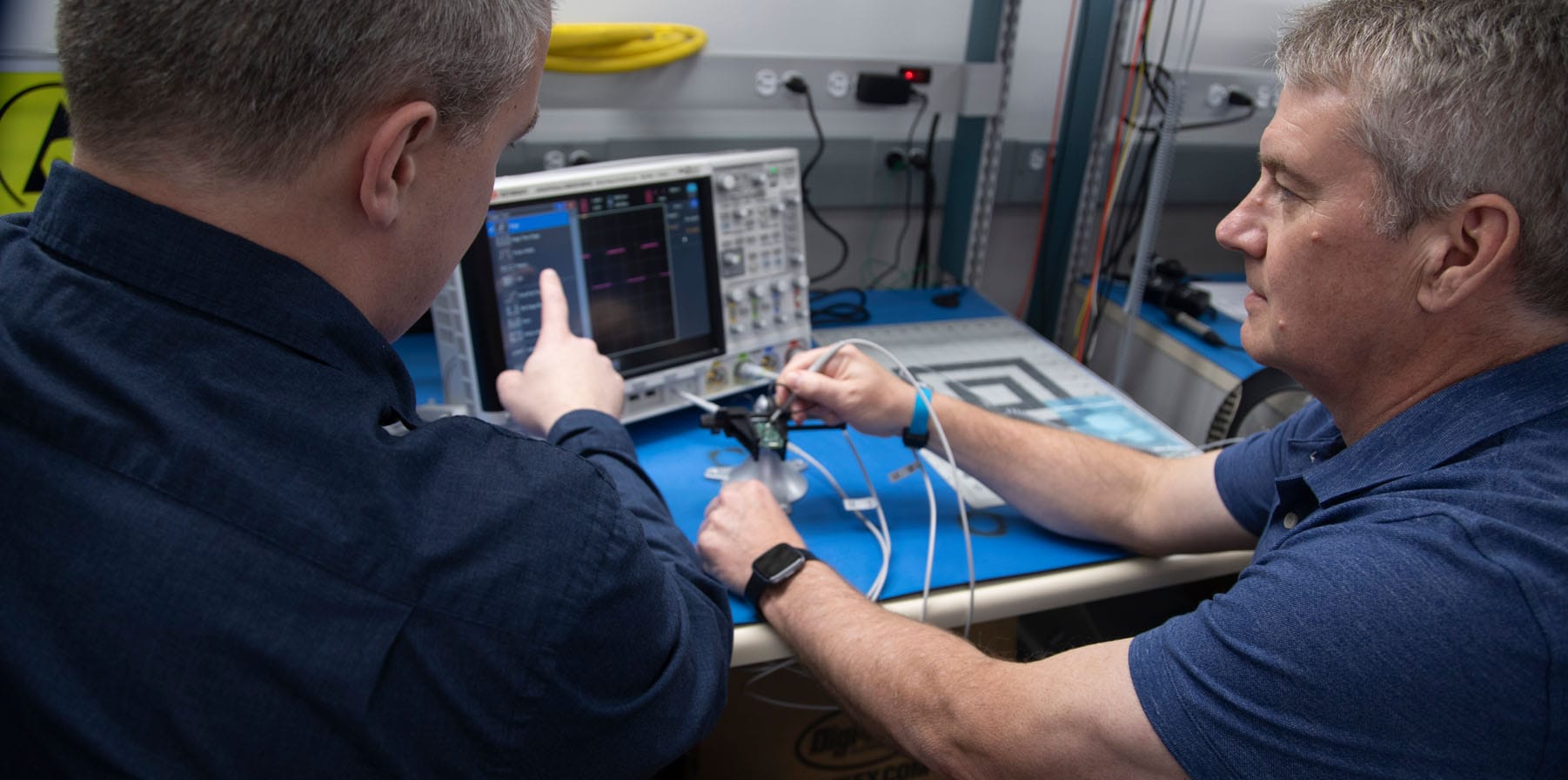
Engineering Expertise from Discovery to Delivery
A collaboration with our R&D team focuses on reducing risk, maximizing investment returns and achieving launch timelines within the complexity of the pharma, medical and in vitro diagnostic device space. Tailored to customers’ unique design, development and manufacturing needs, our engineers offer a complete range of capabilities, services and expertise:
Formulation Development
Formulation and Product Development
With expertise in powder and liquid formulations, as well as a broad portfolio of proprietary device and formulation technologies available to access if required, we can help you overcome the challenges of inhaled formulation development.
Pharmaceutical Analysis
Our large, experienced inhalation group of analytical scientists can undertake the comprehensive array of test methodologies and physical properties characterization required to support the development of inhaled products.

User Experience and Industrial Design
User Experience (UX)
Our UX designers create products that provide meaningful and relevant experiences to users, taking into account the needs of patients, caregivers and providers.
Industrial design and design research
Our team of industrial designers and design researchers focus on creating products that are user-friendly, functional, ergonomic and aesthetically pleasing to patients, caregivers and providers.

Human Factors Engineering
Human Factors Engineering (HFE)
Our HFE experts across the US and Europe plan, execute and document formative and summative usability studies that allow us to streamline the design development process and eliminate the need for late design changes caused by use errors.

Medical Device Engineering Disciplines
Mechanical Engineering
We are renowned for this discipline, which encompasses simulating complex mechanical systems (digital twinning), finite element analysis (FEA), mechanical design, material selection and tolerance analysis. These capabilities help us to reduce the number of components and produce robust designs that meet product requirements.
Electrical Engineering
In pharma drug delivery devices, we leverage our electronics capabilities for motor drives, battery and power management, as well as miniaturization. For our medtech customers, we lean into our colleagues at our parent company, Molex, a global electronics leader.
Fluidics
We are investing in this capability, which is an extension of both mechanical and electrical engineering, especially as it applies to in vitro diagnostic devices.
Software Development, Including Artificial Intelligence (AI)
This capability is evidenced in our reconstitution and lyophilized drug handling software innovations, as well as in our connected bespoke and platform drug delivery devices and cybersecurity services. We also are thoughtfully leaning into AI to drive innovation. Plus, our design centers have extensive experience delivering ISO 62304 compliant software solutions and are mindful and capable of handling foreground and background IP utilization.
Labeling and Package Engineering
Our team of experts helps customers meticulously and cost-effectively design product packaging, while considering the unique needs of medical devices, such as packaging sterilization and ease of use.
Sustainability Engineering
One of our newest R&D competencies, we have hired talent with expertise that allows us to offer customers products with the potential to be more environmentally friendly. This engineering capability allows us to select materials and design products for reusability, refurbishment, recycling and disposability. We also offer expertise in technology and regulatory pathways for more sustainable solutions.
Prototyping
Our ability to deliver rapid prototypes and clinical builds within the walls of our design centers allows us to innovate faster and collaborate across our teams more effectively.

Design Assurance and Quality Engineering
One Quality Management System
Our world-class one Quality Management System, which is consistently implemented across our seven R&D innovation centers and 23 production facilities, ensures that our design and development services meet the rigorous regulatory requirements for medical, drug delivery and in vitro diagnostic devices. Our robust R&D Quality Management Systems include:
- ISO 13485 with Design Controls
- ISO 14971 Medical Devices
- 21 CFR Section 820.30 Design Controls
- European Medical Device Regulation (EU) 2017/745
Regulatory Support
Phillips Medisize offers a range of services to support customers on their path to commercialization, including legal manufacturing, MDR and FDA submission guidance.
Risk Management
By leveraging our expertise in the early stages of design, we can reduce risk in the regulatory and launch timelines, while improving the probability of successful commercialization.
Systems and Test Engineering
Systems Engineering
Ensuring that the pieces come together to a complete winning product. Requirements decomposition, regulatory approaches, standards compliance and regional considerations are only a few of the areas our systems engineers consider.
Test Engineering
Our inhouse team of test engineers help us to characterize and test product performance throughout the development process by developing test methods and fixtures. Leveraging our expertise in testing to ISO 11608 and ISO 20072, our test team helps us to move efficiently through design verification through protocol writing, test execution and reporting.

Design for Volume Engineering
Design for Manufacturability (DfM)
Our DfM capabilities truly differentiate Phillips Medisize R&D because we take it into account from the very first steps of design strategy. Our engineers help customers improve their cost of goods (COGs) and ensure their products are launched on time. Traditional design houses will claim that they can do this step, but we know otherwise. Over the years, we have helped many customers after they have invested considerable resources with a design house for product concepts only to require significant or complete redesign in order to be realized in scaled manufacturing.
Design for Assembly (DfA)
Our DfA capabilities also are unparalleled. Few CDMOs have the engineering expertise to develop devices that can be assembled within design margins. We have dedicated teams focused on delivering a range of services from manual assembly to million-piece automation solutions.
Tool Design and Fabrication
Tooling Engineering
Our engineers across three continents drive tooling strategies, help to design tools at our three tool-building facilities, and offer low-cost, quick-turn tooling.
Assembly Station and Automation
Automation Engineering
Our R&D team collaborates with colleagues across our 23 production facilities around the world to identify appropriate semi-automated or automated solutions to support low-volume and high-volume manufacturing.
Manufacturing
Clinical Trial Production and Supply
Using our in-house manufacturing facilities and equipment, we have the ability to manufacture your drug product (inhalation) and medical devices for use in development studies and clinical trials.

Across Every R&D Step
Program Management
Our team of experienced program managers are the glue across each stage of design and development from initial scoping and contracts to ensuring each device and project is delivered on time and on budget. We never throw programs “over the wall” and our program managers oversee a program through the entire journey.
Engineering Award-winning Design
Our award-winning, human-centered design teams have been frequently recognized for their human-centric, early-stage design and strategy. Our recent awards include:

Featured Resources


