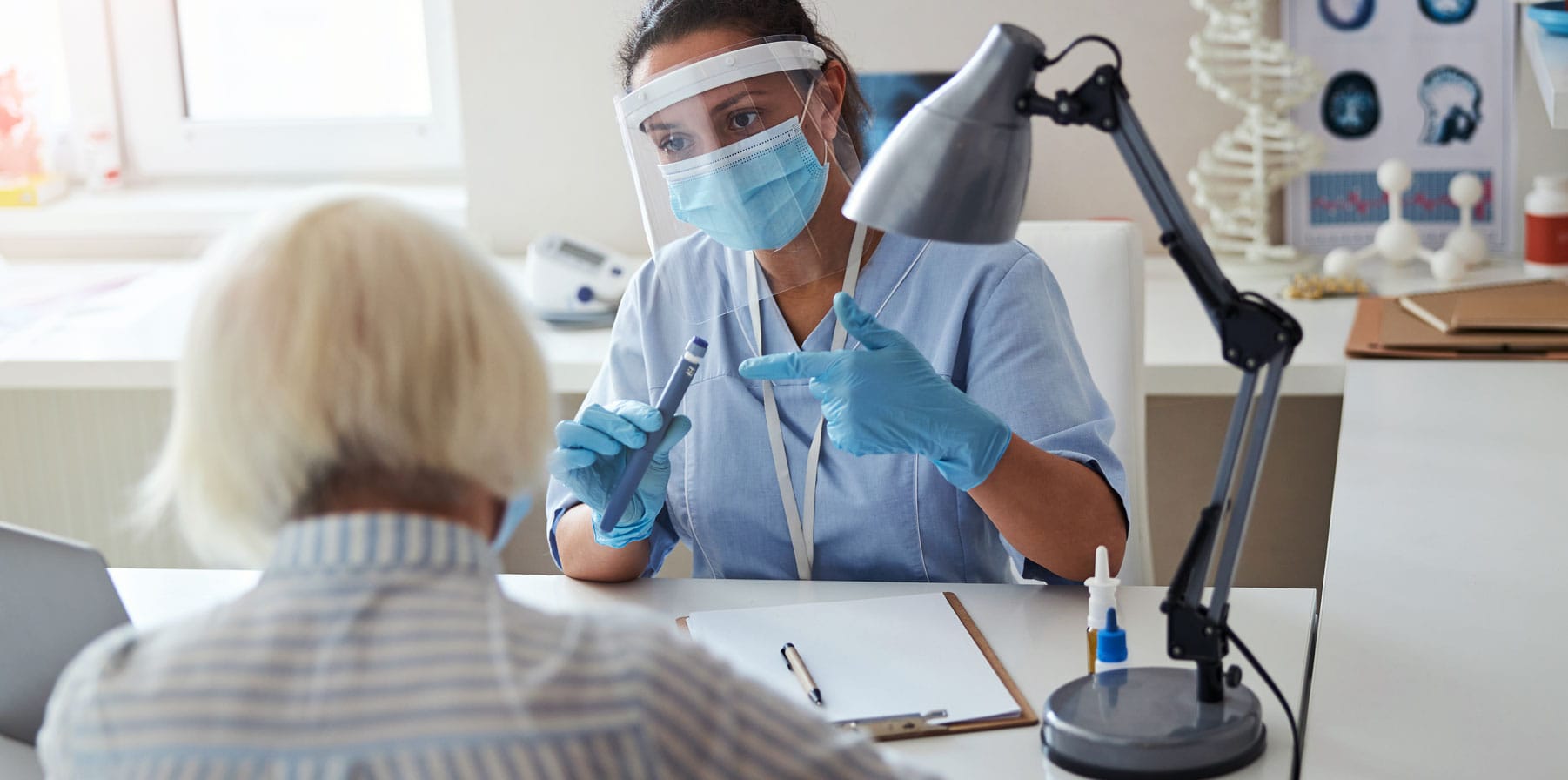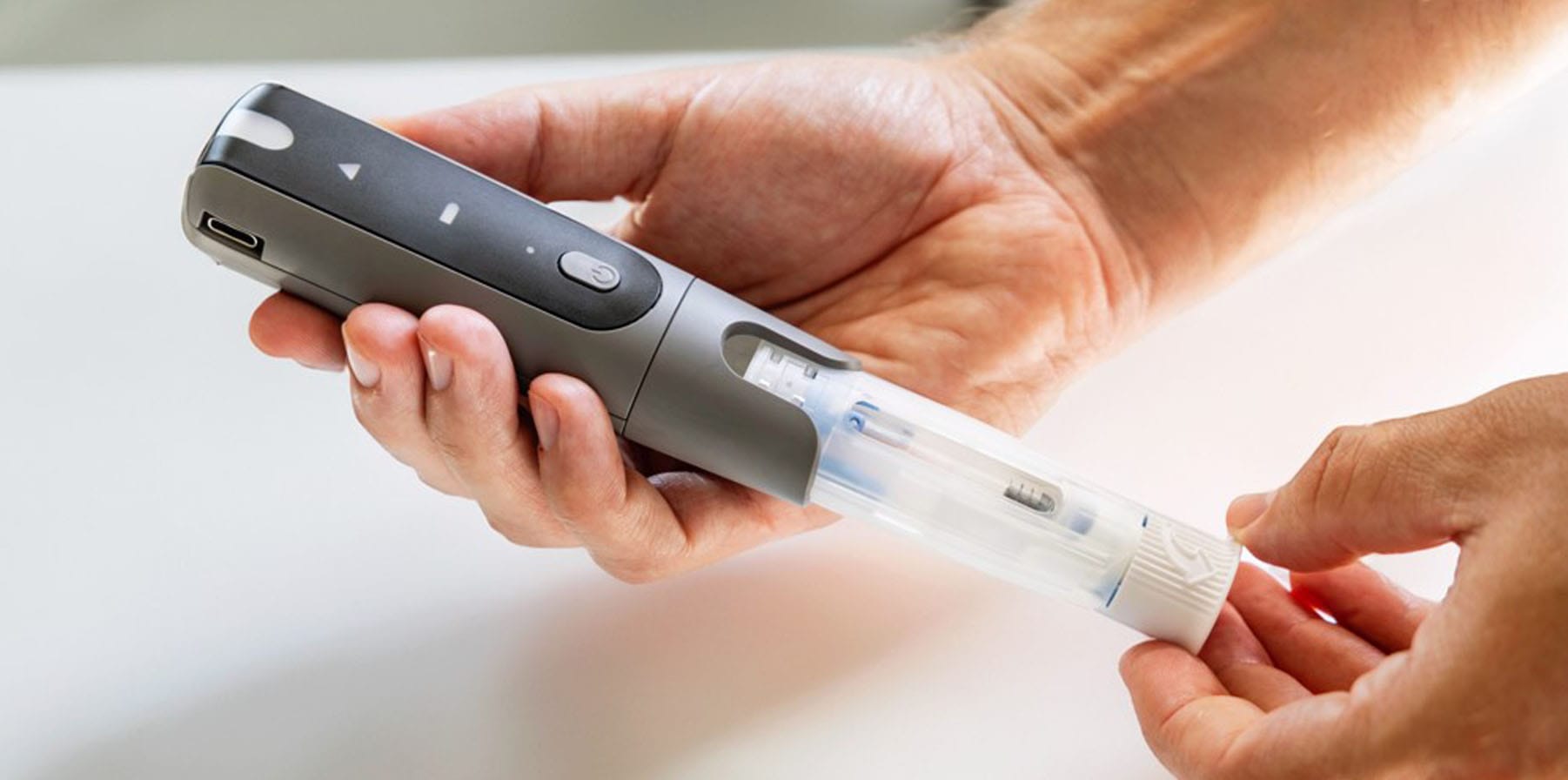
AriaTM Smart Autoinjector
Phillips Medisize is developing an innovative smart autoinjector that is designed to combine the ease of use of a disposable autoinjector with the flexibility of a reusable electronic device.
DESIGNED TO BE

Reusable for environmental stewardship
Ready for the connected world
Powerful and flexible platform for pharma
Small and easy to use
A Reusable Autoinjector with a Disposable Cassette
DESIGNED WITH INTELLIGENT, REUSABLE DRIVE UNIT WITH OPTIMAL CONNECTIVITY
- Powerful motor to drive controlled injection of low- and high-viscosity liquid drugs (1 to 200 cps*)
- Full dose delivery in 10 seconds (adjustable)
- Simple sleeve-triggered/push-action operation
- Visual and audible user feedback
- Rechargeable with 3-year service life (i.e., up to 3 x 365 uses)
- Optional built-in Bluetooth™ low energy (BLE) connectivity
SINGLE-USE DISPOSABLE CASSETTE
- 1 ml and 2.25 ml prefilled syringes (glass or COP) with RNS
- Standard subcutaneous delivery
- Needle safety before/after injection
- Up to 70 percent less CO2e impact than disposable autoinjectors
- Large inspection window
- Optional radio frequency identification (RFID)
Technical Specifications
Data provided for standard models; other options are possible, e.g., higher viscosities with longer injection delivery times.
| 1 ml | 2.25 ml | |
|---|---|---|
| Primary Container | ISO Standard 1 ml long, cut and round flange | ISO Standard 2.25 ml, cut and small round flange |
| Delivery | Up to 1 ml Full, fixed volume | Up to 2 ml Full, fixed volume |
| Injection Depth | Subcutaneous (5.5 mm +/- 1.5 mm) | Subcutaneous (5.5 mm +/- 1.5 mm) |
| Delivery Time | <10 s | <10 s |
| Viscosity Capability | Up to ~200 cps in <10 s (27 g ½ in needle) | Up to ~80 cps in <10 s (27 g ½ in needle) |
| Connectivity Options | Embedded BLE RFID on cassette | Embedded BLE RFID on cassette |
| Needle Safety | Needle shielding pre- and post-use | Needle shielding pre- and post-use |
| Needle Insertion | Manual | Manual |
Designed Around Patients
Designed for extensive audiovisual indications: injection complete signals include dwell time, no manual counting.
The device operates in a similar way to a two-step autoinjector, once the cassette has been inserted; but injection complete signals include dwell time.

Platform Approach
Designed for:
Reusable Device
- Adjustable software to provide an optimized delivery rate
- Only 1 change-part between 1 ml and 2.25 ml models
Cassette Body
- Standard design cassette for both 1 ml and 2.25 ml (subject to PFS type)
- Large viewing window for visibility of drug product
- Space for mandatory labeling
Cassette Cap
- Adjustable to suit a range of rigid needle shields
This product has not obtained a CE marking applicable to the health, safety and environmental regulations of the European Union, and has not been reviewed or cleared for use by the U.S. Food and Drug Administration or comparable health regulatory authorities in other jurisdictions.
*Based on 1 ml delivery in 10 s injection time with 27 g ½ in needle.
Bluetooth is a trademark of Bluetooth SIG, INC.
Featured Resources