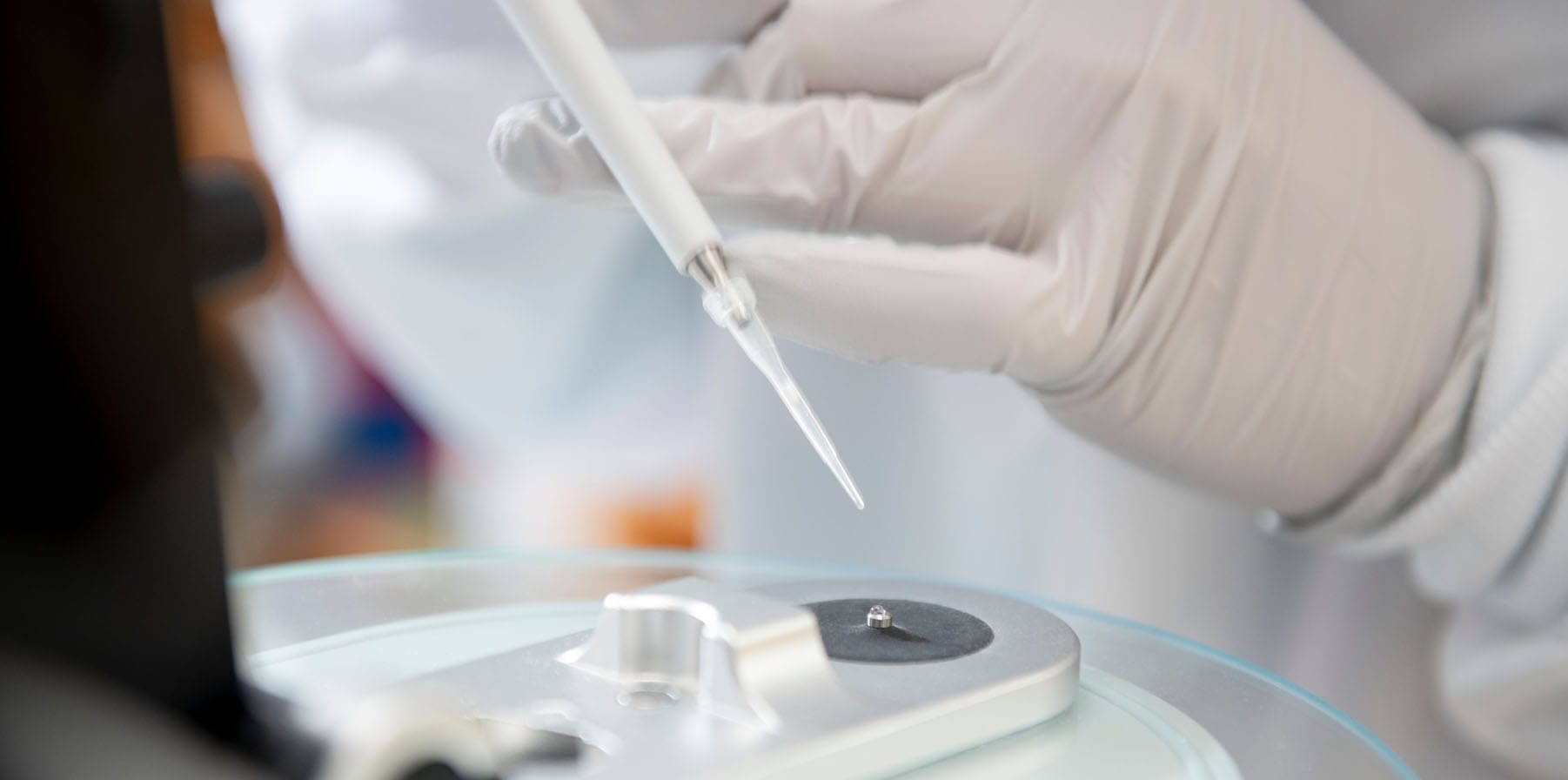
Helping Global Diagnostic Innovators to Design and Manufacture Highly Accurate Devices
From discovery to delivery, the world’s leading in vitro diagnostic (IVD) companies select Phillips Medisize to help them engineer and manufacture consumables and cartridges for point of care (POC) testing, rapid diagnostics, blood collection devices, specimen collection kits and more.
Optimizing the Path for Innovative IVD Devices from Concept to Delivery
As diagnostic companies increasingly invest in promising applications, Phillips Medisize has the capabilities and experience to help address technical and manufacturing challenges for each step of the way, from concept to prototype, and from pilot to production scale manufacturing with:
Mature ISO 13485- compliant and FDA certifications
An integrated design for excellence (DfX) manufacturing strategy, reducing risk, reducing cost, and increasing robustness and throughput from inception
Complex, precision injection molding, welding, sealing and electronic manufacturing capabilities in ISO 7 and ISO 8 clean rooms, providing a one-stop-shop manufacturing solution
Reagent handling solutions, including precision placement and filling, ensuring reagent stability and functionality
Integration of miniaturized electronics, specialty optics components and final device testing, supporting full device assembly
Tailored final packaging options, including management of unique device identification (UDI) and serialization, creating additional value chain efficiencies

Our global footprint and resources help customers develop and scale new products quickly from anywhere in the world.
Designing, Developing and Manufacturing Complex IVD Products
With more than six decades of expertise in design, development and manufacturing, we can support you in every phase of the product life cycle for your point of care, at-home and complex IVD consumables.
We offer flexible manufacturing options for pilot, low-volume and high-volume production. Tailored to your needs, we can help you balance capital investment and production demand, adapting to seasonal or unpredictable demand as well as providing rapid scale-up to meet growing market demand.
We offer a complete range of services and capabilities, including dedicated design and development resources: early-stage design, rapid innovation and optimization of design for manufacturability (DfM) and assembly (DfA), ensuring smooth transfer from prototyping to manufacturing. We also offer liquid and dry chemistry handling for safe, quality-controlled handling and packaging of reagents and diagnostic substances, which help preserve chemistry stability and functionality.
Learn more about our manufacturing capabilities and our reagent handling capabilities.
We Offer Specialized IVD Experience In:
IVD Systems and Components
- IVD consumables for clinical and reference lab instrumentation
- POC systems and consumables
- Microfluidic and macrofluidic cartridges
- Rapid diagnostics
Specimen Collection
- Home collection kits
- Clinical collection kits
- Blood collection devices
IVD Certifications
- ISO 13485 and 21 CFR 820 201/211 controls
- National regulatory compliance in applicable countries
Featured Resources


