Microassembly for IVD Cartridges & Consumables
At Phillips Medisize, our precision microassembly capabilities enable us to meet complex miniaturization needs when manufacturing IVD cartridges that require critical accuracy and reliability. Our experience supports the development and manufacture at scale of smaller, smarter and more sophisticated medical technologies that help enable healthier patient outcomes.
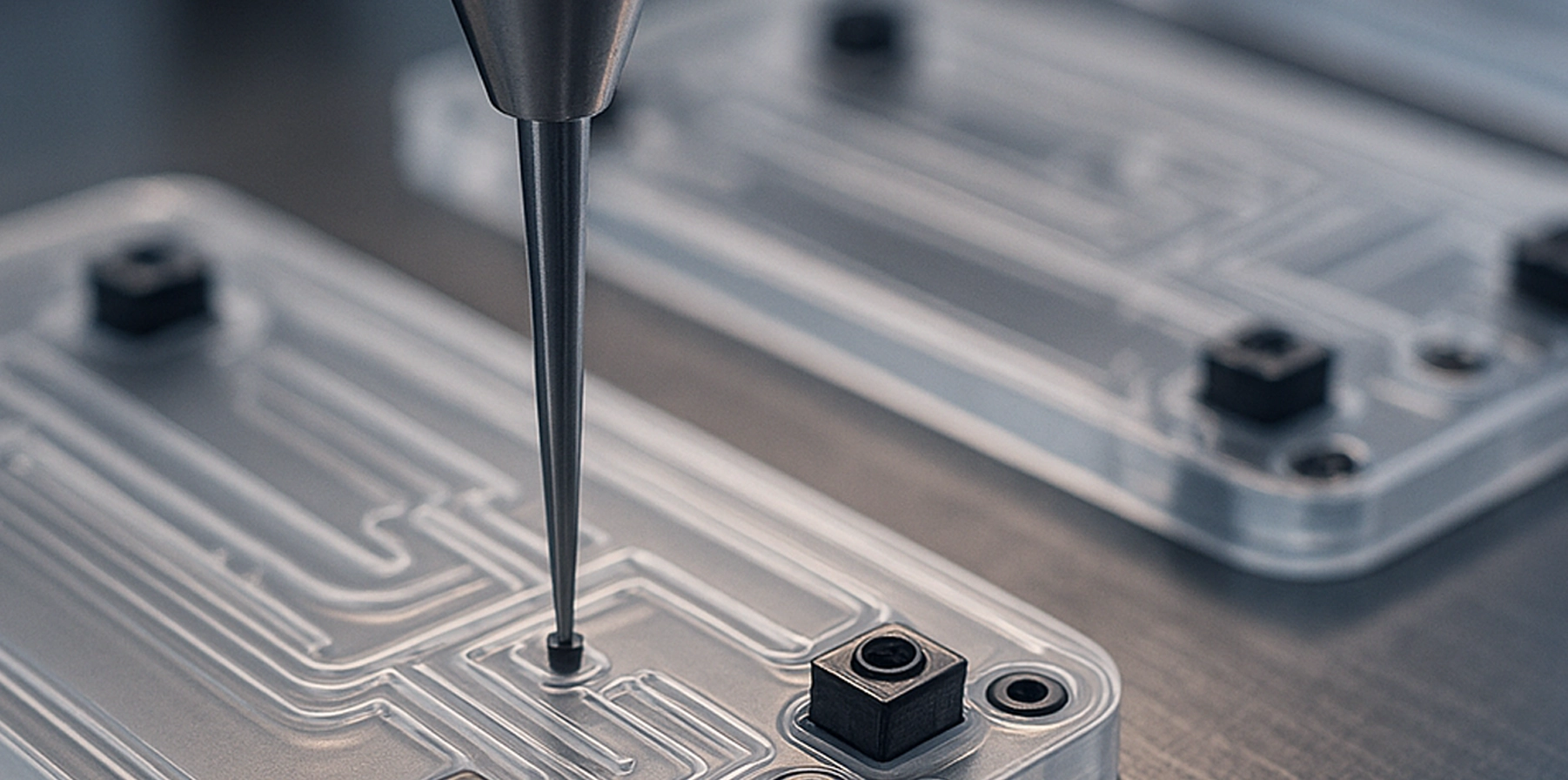
Microassembly allows the integration of miniature components with strict tolerances and exacting quality standards. This capability is crucial for medical devices such as IVD cartridges with integrated biosensors that demand high performance in compact designs.
Key IVD Applications
Our microassembly abilities support a range of IVD use cases, including:
- IVD cartridges with biosensors: Reliable integration of microfluidic channels and sensors enabling excellent test sensitivity and specificity
- Point-of-care testing: Miniaturized fluid handling enabling faster turnaround time and near patient results
- Molecular diagnostics: Precision assembly of fluidic and sensor components where biomolecule handling and contamination control are essential
- Complex consumables for testing and sequencing: High-volume automated manufacturing for disposable PCR testing, isothermal NAAT and NGS cartridges
Complex Assembly and Integration
We are knowledgeable in the precision microassembly of electrical and fluidic interconnects critical to advanced medical devices, and we specialize in fully automated precision manufacturing, assembly and bonding of components requiring liquid or gas tightness, such as for microfluidic cartridges, sample collection devices and specimen preparation modules for IVD platforms. Additionally, we offer a proprietary manufacturing process that enables the seamless integration of fluidic, electrical, electronic and mechanical components within a single, multilayered structure. Our portfolio of solutions meets the demanding requirements for performance and durability in a range of IVD consumables.
Advanced Microassembly Manufacturing
- Automated Microassembly Lines: Sophisticated automation for consistent and scalable production
- Precision Robotics: High-accuracy robotic placement, bonding and joining technologies
- Cleanroom Environments: ISO-classified cleanrooms Class 7 and 8 providing contaminant control and ensuring a particle-free manufacturing and assembly process
- Specialized Joining Techniques: Welding (laser, ultrasonic, micro), soldering, bonding (solvent, thermal fusion) and adhesive dispensing
- Advanced Inspection: Vision systems and microscopy for rigorous quality control

Manufacturing Key Processes
- Component handling and feeding optimized for microscale parts
- High-precision alignment to ensure perfect assembly
- Monitoring and logging of bonding method parameters to ensure process consistency, enable real-time quality control and support full traceability for regulatory compliance throughout manufacturing. Seamless integration with other manufacturing processes
Materials Expertise
- Diverse Materials Handling: Including plastics, metals, and flexible and printed circuits (FFC/FPCs) tailored for medical device requirements
- Reagent Deposition: Lyophilized bead handling and singulation, spotting, precision liquid handling.
Benefits of Our Microassembly Expertise
- Consistent Quality: Superior repeatability with minimal defects
- High Manufacturing Yields: Deliver competitive cost of goods sold (COGS) for our clients while maintaining uncompromised device reliability and performance
- Device Miniaturization: Enabling smaller, more efficient and reliable high-performance device design
- Faster Time-to-Market: Scalable processes to support clinical trials through commercial production
- Regulatory Compliance: ISO 13485 certification, and registration and compliance with major global regulatory standards, including FDA (United States), MDR and IVDR (European Union), and other relevant requirements established by regulatory authorities worldwide
Quality and Compliance
Our facilities maintain a stringent Quality Management System (QMS) certified to ISO 13485. All assemblies undergo in-process and final inspections with comprehensive documentation and traceability to meet the most rigorous regulatory requirements for IVD devices. We have extensive experience with regulatory agencies (FDA, EMA, MHRA), and we adhere to the standards and regulatory requirements aligned with the products and countries in which we operate or distribute. Our QMS is designed with flexibility to meet both customer needs and regulatory demands, and we ensure compliance with all applicable regulations in MDSAP countries (Australia, Brazil, Canada, Japan and the US), China and other markets where products we manufacture are available.
While it may seem that most medical CDMOs are similar when comparing the lists of certifications, accreditations and registrations, Phillips Medisize is different in all the best ways possible, including one QMS synchronized across all our global production sites.
Featured Resources


