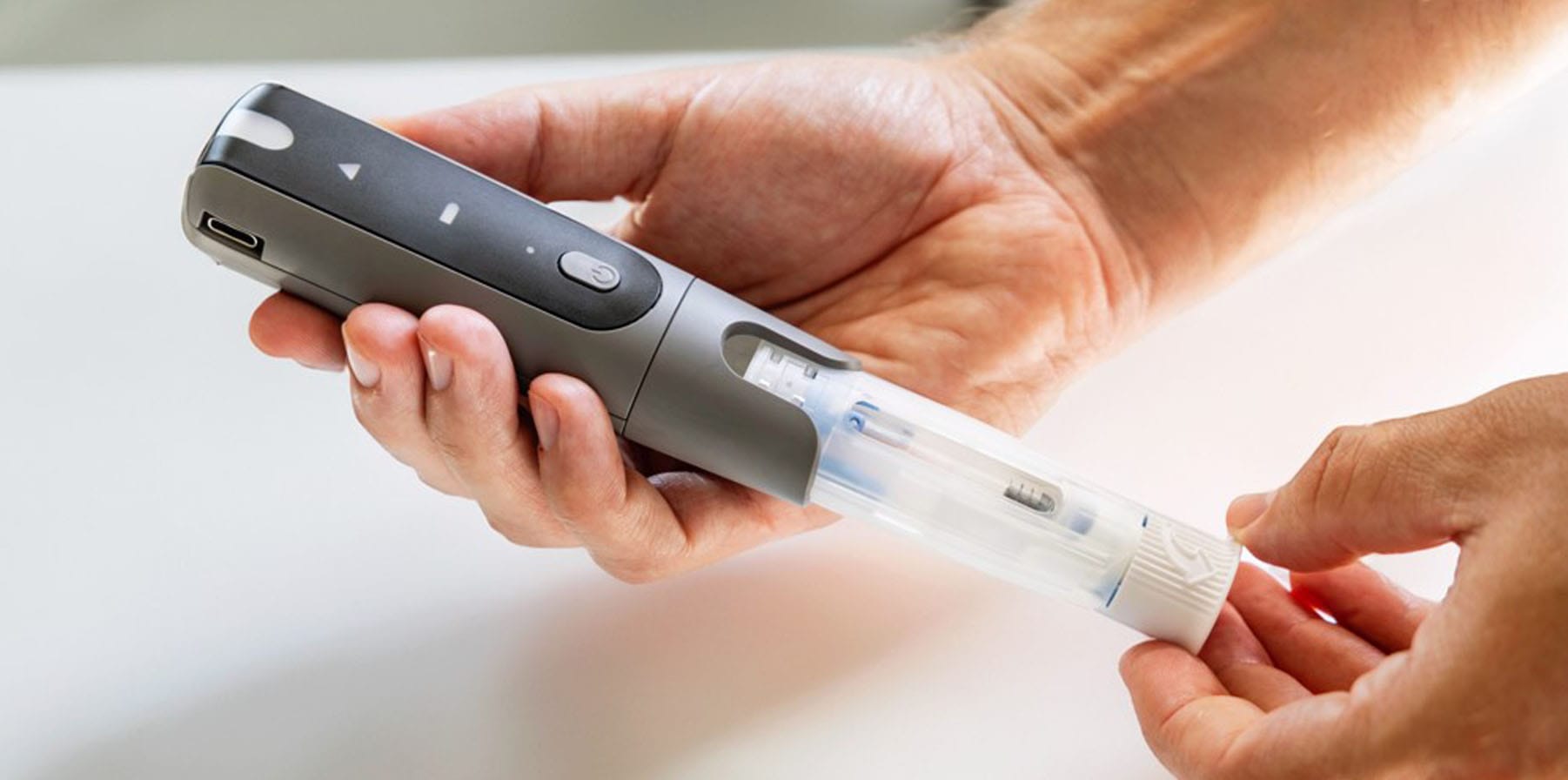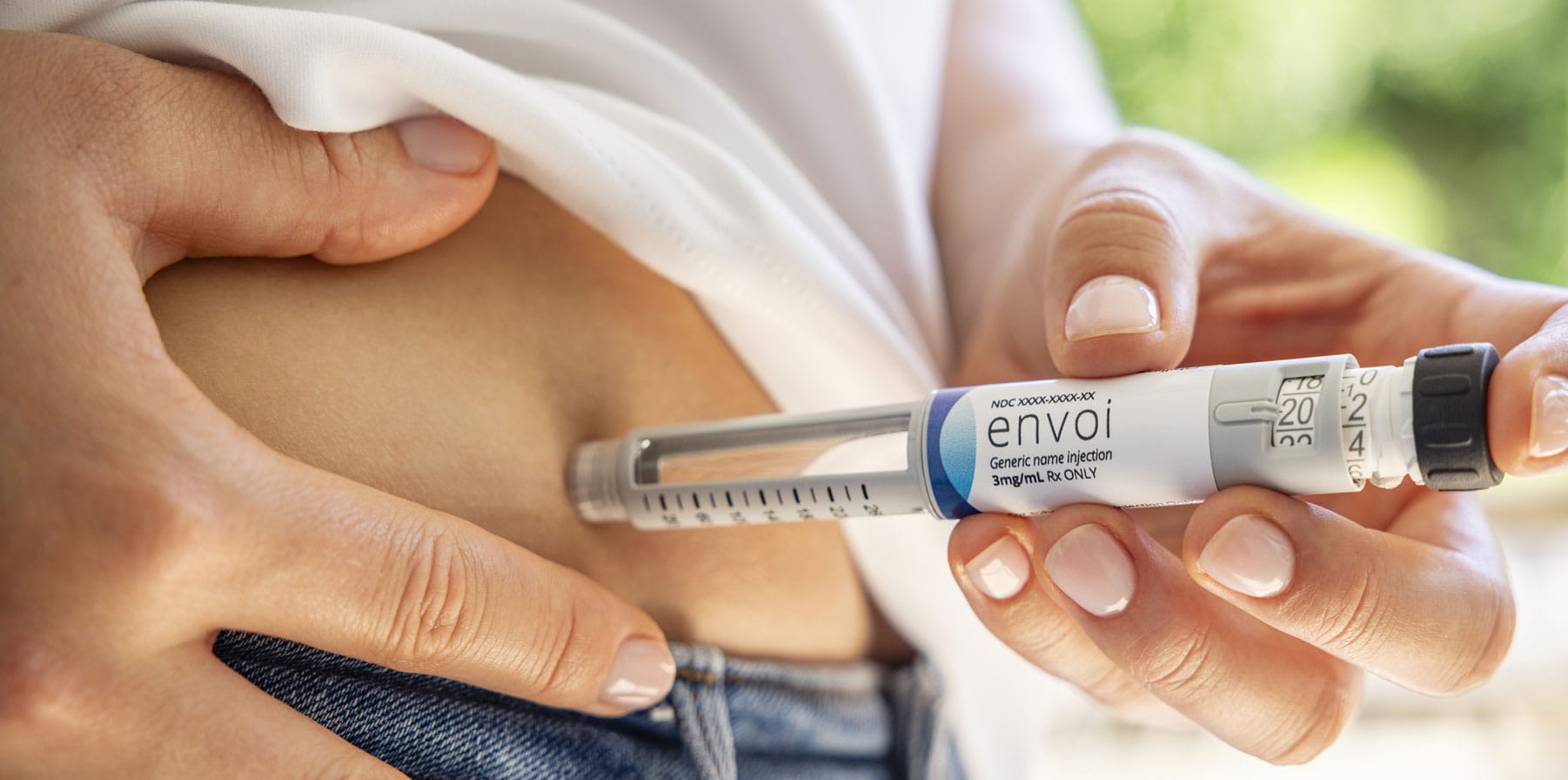
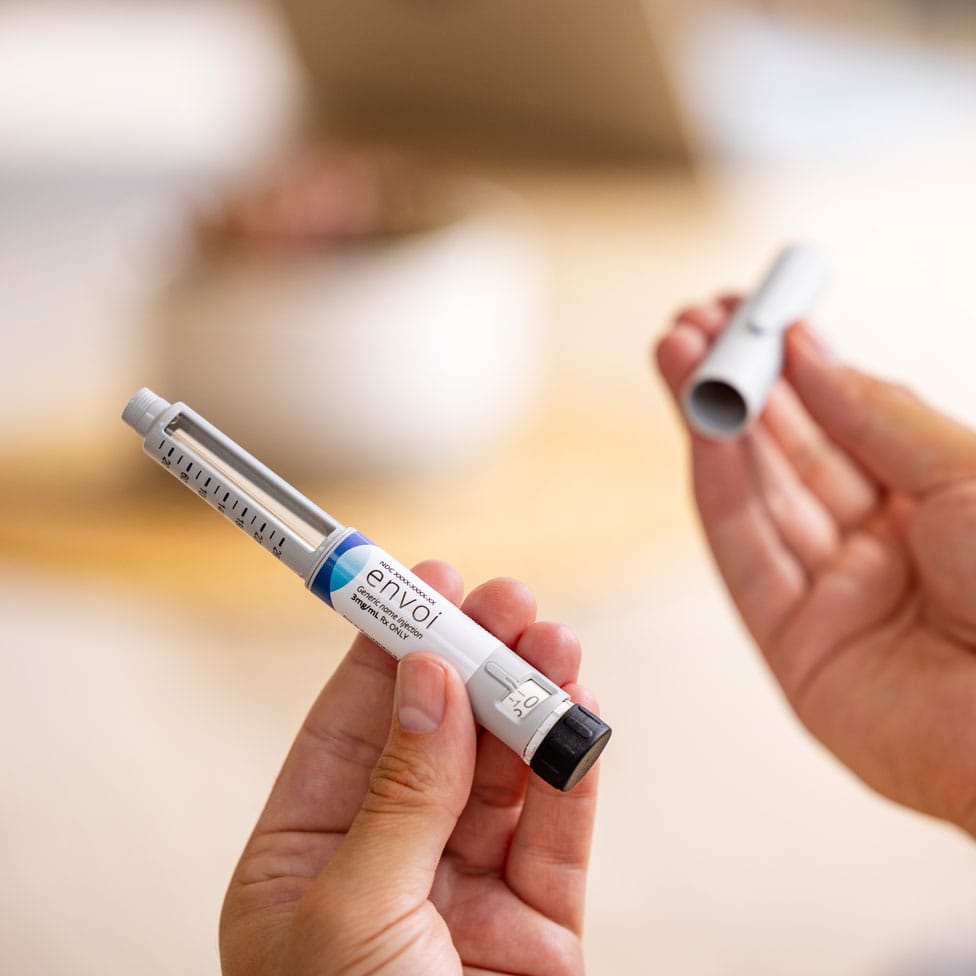
A Familiar Pen Injector at an Affordable Price
The EnvoiTM Pen Injector is a compact and user-friendly pen device designed for the subcutaneous delivery of medication. It is intended to be used for self-administration of injectable medications, including insulin and glucagon-like peptide-1 (GLP-1). The Envoi Pen Injector is an important addition to the Phillips Medisize portfolio, empowering biopharma companies to accelerate the roll out of novel and generic drug treatments with significant economies of scale.
This solution provides the advantages expected from familiar pen designs, while benefiting from decades of our device development expertise and large-volume manufacturing capabilities to reduce the challenge of bringing affordable drugs and delivery devices to market.
Envoi Pen Injector
Offering access to a pen that aligns with cutting-edge devices available today, at an appealing price point, while reducing the cost, time and risk associated with a custom development:

Designed for 3.0 ml and 1.5 ml cartridges

An easy-to-scale platform, currently available for 3 ml cartridges

Designed for patients managing diabetes, growth hormones, fertility and osteoporosis
Benefits of the Envoi Pen Injector
- Combining a compact design with the capability of administering up to 80 IU in a single injection (~152 mm length)
- Featuring a limited elongation (~33 mm) for maximum dosing
- Supporting ease of administration for individuals with small hands or dexterity issues
- Incorporating a flexible design to accommodate fixed and variable dosing requirements
- Targeting relatively low dosing force*
- Handling final assembly for design validation testing (DVT) and production of clinical batches
Backed by Our Proven Experience in
High-Volume / High-Quality Manufacturing

Established Global Footprint Across Three Continents
Device Specifications
A User-Friendly Patient Experience
Supporting Your Product Journey, Every Step of the Way
Our comprehensive technical documentation package helps support your U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) submissions. We can provide:
- Technical components’ specifications for development of pen and cartridge assembly lines
- Part build services for stability studies
- Technical dossier for reference in your drug approval applications
- Technical files for pen customizations, if applicable
- Regulatory support in different geographical regions
*Depending on the cartridge specification – the DVT to assess the actual force
This product has not obtained a CE marking applicable to the health, safety and environmental regulations of the European Union, and has not been reviewed or cleared for use by the U.S. Food and Drug Administration or comparable health regulatory authorities in other jurisdictions.
Featured Resources